NGS DATA ANALYSIS: A COMPREHENSIVE GUIDE FOR LIFESCIENCES STUDENTS
Next-Generation Sequencing (NGS) has transformed genomics by enabling large-scale DNA and RNA sequencing, but extracting meaningful insights requires systematic data analysis. This blog provides MSc students with a comprehensive overview of NGS workflows, from quality control and read preprocessing to alignment, post-alignment processing, variant calling, and RNA-seq analysis. It highlights key tools such as FastQC, Trimmomatic, BWA, STAR, GATK, and DESeq2, while emphasizing the importance of reproducibility, data visualization, and pathway analysis. Common challenges like data volume, batch effects, and evolving genome annotations are discussed, along with suggested hands-on exercises to build practical skills. By mastering each step, MSc students can confidently translate raw sequencing data into reliable biological insights and prepare for careers in genomics, bioinformatics, and biomedical research.

Next-Generation Sequencing (NGS) has become a cornerstone in molecular biology, genomics, and bioinformatics. For MSc students entering this field, understanding both the theoretical concepts and practical workflows of NGS data analysis is essential. In this guide, we delve into the key principles, tools, and hands-on practices that form the backbone of NGS analysis, providing a clear path from raw sequencing data to meaningful biological insights.
Why NGS Data Analysis Matters
NGS enables researchers to explore the genome and transcriptome at unprecedented resolution. It allows the detection of genetic variants that may contribute to diseases, the study of gene expression patterns in both normal and diseased states, the exploration of microbial diversity in metagenomic samples, and the investigation of epigenetic modifications through techniques such as ChIP-seq and ATAC-seq. For MSc students, mastering NGS analysis not only enhances research skills but also opens opportunities in genomics labs, biotechnology companies, and computational biology projects.
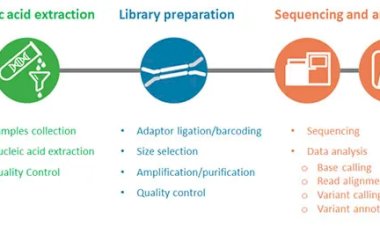
Quality Control (QC)
Before performing any downstream analysis, assessing the quality of raw sequencing data is crucial. Tools such as FastQC provide detailed reports on per-base sequence quality, GC content, sequence duplication levels, and the presence of adapter contamination. For multiple samples, MultiQC can aggregate these reports, allowing for a quick overview of overall data quality. MSc students should pay attention to unusual GC content or high duplication rates, as these may indicate contamination or artifacts introduced during library preparation or sequencing. Conducting rigorous quality control at the start ensures that subsequent analyses are reliable.
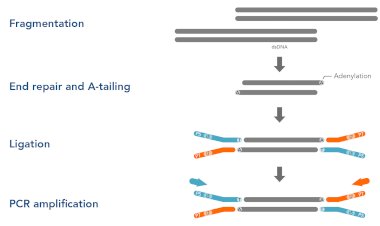
Read Preprocessing
NGS reads often contain adapter sequences, low-quality bases, or sequencing errors that can negatively impact downstream analysis. Preprocessing involves trimming adapters and low-quality sequences using tools like Cutadapt or Trimmomatic, and filtering out reads that are too short or of poor quality. Maintaining a record of the number of reads removed during preprocessing is highly recommended, as it aids troubleshooting and ensures transparency in the analysis workflow. Proper read preprocessing is essential for accurate mapping and reliable results in later stages.
Alignment / Mapping
Once reads are preprocessed, they are aligned to a reference genome to determine their genomic positions. DNA sequencing reads are typically aligned using tools such as BWA or Bowtie2, while RNA-seq reads require spliced aligners like STAR or HISAT2 to account for exon-exon junctions. Accurate alignment is critical because errors in mapping can propagate through the analysis pipeline, affecting variant detection and gene expression quantification. MSc students should be mindful of alignment metrics, including the proportion of mapped reads and coverage uniformity, to assess the success of this step.
Post-alignment Processing
After alignment, further processing ensures that the data are ready for variant calling or expression analysis. This includes sorting and indexing alignment files, removing duplicate reads using tools like SAMtools or Picard, and performing base quality score recalibration, particularly for DNA variant calling, using GATK BaseRecalibrator. Monitoring read depth and coverage at this stage is important, as insufficient coverage can reduce confidence in variant calls or gene expression estimates. Attention to these details helps maintain the accuracy and reliability of downstream analyses.
Variant Calling (DNA-seq)
For DNA-seq datasets, the identification of single nucleotide polymorphisms (SNPs), insertions, deletions, and structural variants is a key objective. GATK HaplotypeCaller is considered a gold standard for germline variant calling, while VarScan or FreeBayes may be used for pooled or somatic samples. MSc students should also learn to filter variants using VCFtools or BCFtools, applying criteria such as read depth, quality score, and allele frequency. Proper variant calling and filtering are crucial for generating high-confidence results that can be used in further functional or clinical interpretation.
RNA-seq Analysis
RNA-seq enables comprehensive transcriptome profiling, allowing researchers to study gene expression under various biological conditions. The first step is quantification, where tools like FeatureCounts or HTSeq-count are used to count reads mapping to each gene, producing a matrix of raw counts. Next, normalization methods such as TPM, FPKM, or the built-in approaches in DESeq2 are applied to account for differences in sequencing depth and library size. Following normalization, differential expression analysis identifies genes that are significantly up- or down-regulated between conditions using tools like DESeq2 or edgeR. MSc students should also leverage Principal Component Analysis (PCA) plots to visualize sample clustering, detect batch effects, and ensure that observed differences reflect true biological variation rather than technical artifacts.
Functional Annotation and Pathway Analysis
After identifying genes or variants of interest, it is important to interpret their biological significance. Gene Ontology (GO) enrichment analysis highlights overrepresented biological processes, while KEGG pathway analysis provides insight into affected metabolic or signaling pathways. Network-based analyses, such as those using the STRING database, can reveal protein-protein interactions and functional relationships between genes. For MSc students, these analyses provide a bridge between raw data and meaningful biological conclusions, helping to generate hypotheses for further experimental validation.
Visualization
Effective visualization is key to communicating NGS results. Tools like IGV (Integrative Genomics Viewer) allow detailed inspection of alignments, variants, and gene structures. R and Bioconductor packages enable the creation of heatmaps, volcano plots, and PCA plots, while Circos plots are particularly useful for visualizing genomic rearrangements. MSc students should recognize that well-designed visualizations not only support scientific conclusions but also enhance the readability and impact of publications or presentations.
Challenges and Considerations
NGS data analysis comes with challenges that must be carefully managed. The sheer volume of sequencing data often requires high-performance computing clusters or cloud-based resources. Reproducibility is critical, and using workflow managers like Snakemake or Nextflow can help automate pipelines and track analysis steps. Batch effects, particularly in RNA-seq, can obscure true biological signals, so careful experimental design is essential. Additionally, reference genomes and gene annotations are regularly updated, so documenting the versions used is necessary for reproducibility and comparison across studies.
Suggested Hands-On Exercises for MSc Students
MSc students can gain practical experience by performing key NGS tasks. Running FastQC and MultiQC on a small RNA-seq dataset helps develop quality assessment skills. Adapter trimming and read filtering using Trimmomatic provides experience in preprocessing. Aligning reads to a reference genome using STAR or BWA teaches mapping principles. Variant calling and annotation with VCFtools and SnpEff develops expertise in genomic interpretation. Finally, performing differential expression analysis and visualizing results in R reinforces the full RNA-seq workflow from raw data to biological insight.
IBRI Noida: Advancing NGS Data Analysis Training
The Indian Biological Sciences and Research Institute (IBRI) in Noida offers specialized training in NGS data analysis, focusing on computational techniques and bioinformatics tools. Their hands-on programs cover the entire NGS workflow, from quality control and sequence alignment to variant calling and functional annotation. IBRI's training emphasizes the use of industry-standard tools and pipelines, helping students and professionals develop proficiency in NGS data analysis workflows. By simulating real lab environments in a dry lab setting, the institute equips trainees with the confidence and practical knowledge needed to tackle genomic data challenges in research and clinical contexts. This training is ideal for individuals from diverse backgrounds who want to enter the fields of genomics, bioinformatics, and personalized medicine, serving as a valuable bridge between theoretical knowledge and applied expertise.
Conclusion
NGS data analysis is both challenging and rewarding. MSc students who develop proficiency in the end-to-end workflow—from raw reads to meaningful biological insights—are well-prepared for careers in genomics, bioinformatics, and biomedical research. Mastery of NGS analysis requires practice, thorough documentation, and intellectual curiosity, all of which are essential for transforming complex sequencing data into actionable scientific discoveries.